Join the world's largest real-world PRP & Tendon study
Be part of an international, multicenter, scientifically-driven project to transform clinical practice into solid evidence
Over 3,000 patients
100+ international centers
Be part of the solution
Join us
THE SCIENTIFIC CHALLENGE
Why this matters
Chronic tendinopathies afflict millions of patients worldwide, yet the rapid adoption of platelet-rich plasma
(PRP) treatments still outpaces the evidence base: protocols vary widely, product characterization is inconsistent, and follow-up metrics are rarely harmonized. By capturing and analysing large-scale, real-world data across multiple centres, this project delivers the rigorous, standardised evidence needed to turn today’s heterogeneous practice into globally aligned, data-driven care.
Patellar Tendinopathy
Achilles Tendinopathy
Epicondylitis
Plantar Fascitis
study highlights
The largest Real-World PRP Study ever conducted
More than 100 centers worldwide.
Over 3,000 patients.
Real clinical practice.
Minimal disruption to your daily workflow.
Real World Evidence
Real World Evidence (RWE) design: fully integrated into your clinical routine.
PRP Characterization
PRP hematological characterization via automated hematology analyzers
Artifitial Intelligence
AI-powered data analysis identifying predictive response profiles
Scientific impact
High-impact scientific publications planned
Meet the clinical promoter
This international real-world study is coordinated by Dr. Ferrán Abat, one of the world’s leading experts in tendon pathologies and regenerative medicine.
- Medical Director at ReSport Clinic (Barcelona, Spain)
- International authority in PRP application for tendon injuries
- Extensive experience in multicenter clinical research
- Over 100 peer-reviewed scientific publications in musculoskeletal disorders
- Active contributor to major scientific societies and international consensus guidelines
This study represents a unique opportunity to finally generate
large-scale, real-world evidence that can drive global
clinical guidelines for PRP treatments in tendon pathologies

HOW YOUR PARTICIPATION WORKS
Your Participation: Easy, Valuable, Impactful
You continue
treating patients as usual
Register each case in less than 4 minutes using
BioSmartData®
platform
Our platform structures, protects and analyzes your
data securely
Independent, ethically approved,
non-commercial study
Co-authorship opportunities and full scientific visibility
Apply as Investigating Center
The clinical kickoff starts July 2025
Founding centers will lead international recruitment, gain early co-authorship,
and become reference sites for global scientific leadership.
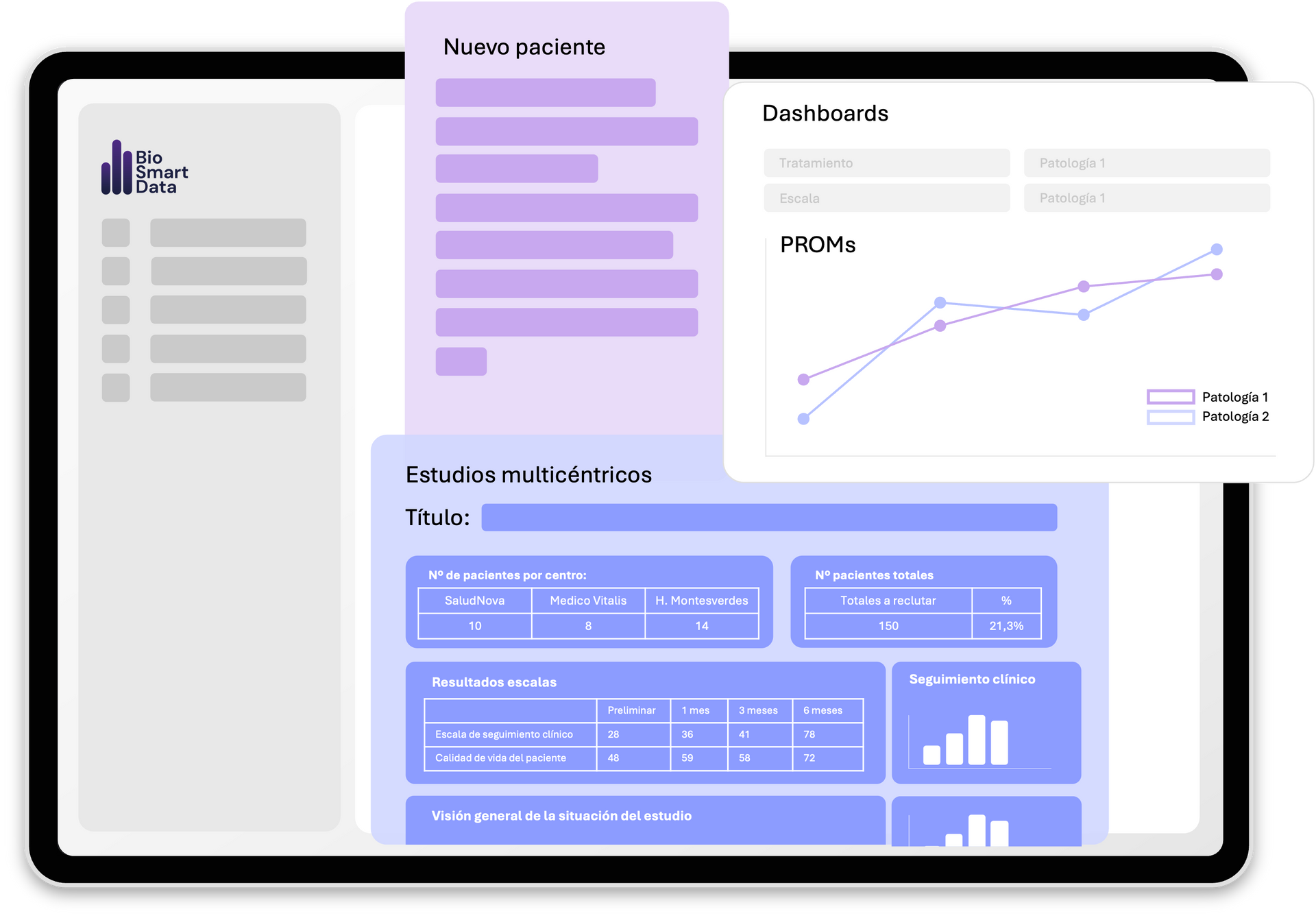
THE TECHNOLOGY BEHIND
Powered vy BioSmartData Technology
- Fully digital eCRD powered by BioSmartData®
- Direct connection to hematology analyzers for PRP classification
- Data encryption, GDPR-compliance & ENS security levels
- AI-powered analysis to identify predictive clinical variables after the study
SIMPLE PROCESS:
Patient → PRP Extraction → Digital Data Entry → Secure Analytics → Scientific Outcomes
FAQs
Who can participate?
Any clinical center, hospital or private practice with experience in PRP (Platelet-Rich Plasma) treatments applied to tendon pathologies (patellar, Achilles, epicondylitis, plantar fasciitis). Both public and private institutions are welcome.
What is the cost to participate?
Participation in the study itself is free of charge. Centers only assume the costs associated with acquiring the technology required to operate:
• The BioSmartData® software license
• A compatible hematology analyzer for PRP characterization.
What technology do I need to participate?
• A valid BioSmartData® Software license (cloud-based, easy installation).
• A hematology analyzer capable of PRP parameter analysis (platelet count, WBC, RBC, volume, etc.), compatible with the BioSmartData® system.
• Internet connection to access the platform securely.
How long is the follow-up period for each patient?
Each patient will be monitored for a minimum of 12 months following their PRP treatment, allowing us to collect both short- and mid-term clinical outcomes.
What type of data is collected?
The platform captures structured clinical data directly from your routine practice, including:
• Patient demographics (fully anonymized)
• Clinical diagnosis and tendinopathy location
• PRP preparation parameters and hematology results
• Treatment protocols used
• Functional outcome measures and clinical scales
• Follow-up assessments at predefined timepoints
Who owns the data?
All data is fully anonymized and managed under strict ethical standards. The study database is a collective scientific repository governed by the international research consortium and scientific committee.
Each participating center maintains access to its own cases, and global aggregated data will be used for joint scientific publications.
How is patient data protected?
Patient privacy and data security are top priorities. The platform ensures:
• Full data anonymization
• AES-256 encryption protocols
• GDPR compliance (Europe)
• ENS High Level Security (Spain)
• Controlled access only for authorized research staff
Become part of the global evidence. Join today
Have more questions? Feel free to contact us >





